MIGS
Microinvasive Glaucoma Surgery
The acronym MIGS refers to a group of glaucoma surgeries that lower IOP through small incisions and improve the natural outflow pathways, as opposed to bypassing the drainage system which occurs with traditional glaucoma surgery. Traditional glaucoma surgery has a higher reward in more likely achieving the lowest IOP possible with surgery; however, there also is higher risk with traditional bypass surgery. The main, although infrequent, feared risk with traditional surgery is achieving to low of IOP thus exposing the patient to vision-threatening complications. Although MIGS procedures typically do not produce as dramatic of IOP (pressure) reduction, the risk of achieving to low of IOP is virtually impossible with MIGS procedures.
Types of MIGS
Trabectome
A new minimally invasive surgical procedure called Trabectome® for treatment of open-angle glaucoma. Many people have benefited from this safe and effective treatment. When medication does not bring eye pressure down, Trabectome is often an excellent treatment option. Trabectome is a breakthrough procedure that successfully lowers intraocular pressure in diseased eyes 80 percent of the time. During the minimally invasive surgery, the surgeon makes just one small incision. Using the Trabectome, a specially designed device, the surgeon removes tissue debris that blocks the flow of fluid in the eye. The procedure is performed on an outpatient basis using local anesthesia. It just takes one suture, if any, to close the incision and best of all – the procedure takes less than 10 minutes on average!
The risk of infection and post-operative complications is significantly lower than in traditional glaucoma procedures. Additional benefits include:
- No pain. Patients do not report significant discomfort during or after the procedure.
Minimally invasive. A small incision means less pain and quicker recovery. - High success. Improved eye health and function occur in 80% of patients.
- Precision. Specially designed surgical instruments enhance accuracy.
- Safety. It’s approved by the FDA and Dr. Stiles has completed thorough clinical training prior to making the procedure available. He currently trains other surgeons from around the country on this procedure.
- Flexible. The procedure can be combined with cataract extraction.
- Cost-effective. Patients’ need for glaucoma management medication usually drops dramatically. Our goal is always to maximize health care resources.
Ab-Interno Canaloplasty (ABiC):
This procedure involves making a microscopic incision into the drainage tissue and inserting a catheter within the drainage canal. The catheter is advanced for 360 degrees into the entire drainage canal. The catheter is then withdrawn while injecting an expansive and clear substance (viscoelastic) throughout the entire drainage system. By breaking down adhesions and blockages within the drainage system, fluid drainage is improved, and the IOP is improved. This can be performed with cataract surgery or as a stand-alone procedure.
Gonio-Assisted Transluminal Trabeculotomy (GATT):
GATT is most successful in glaucoma which developed at an unusually young age (Juvenile Glaucoma), however recently has been shown successful in open angle glaucoma in other ages as well. Similar to ABIC, a catheter is used to intubate the entire drainage canal. However, with GATT, the catheter is removed in a way to cut through the maldeveloped drainage tissue to produce more direct outflow of eye fluid downstream.
CyPass:
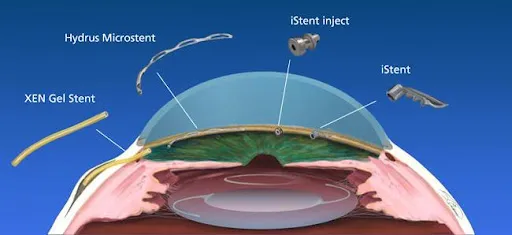
CyPASS was FDA approved for use with cataract surgery in mild to moderate open angle glaucoma in November 2016. (illustration) CyPASS is a surgical device which is implanted in the suprachoroidal space. This space is normally responsible for about 20% of the eye’s natural outflow system. CyPASS enhances this outflow pathway thus lowering IOP. Similar to iStent, it only has insurance coverage when combined with cataract surgery and not as a stand-alone procedure.
iStent
Although FDA approved in June of 2012, SEE was involved years prior in the multi-center research study which led to its approval. The iStent is currently the smallest implant known to be placed in the human body. Under high magnification the device is delicately placed in the drainage angle to bypass ocular fluid around the diseased trabecular meshwork outflow tissue directly into the less diseased tissue downstream. Unlike traditional glaucoma surgery, the surgeon is NOT bypassing the entire outflow system thus not exposing the patient to the higher risks of traditional surgery.
In 2012, Glaukos, the company which developed the iStent, declared SEE as one of 12 MIGS Centers of Excellence within the US. These locations were chosen as centers trusted to train other eye surgeons in performing the iStent procedure to the company’s standards.
